Abstract
Introduction: Relapsed or refractory (r/r) acute myeloid leukemia (AML) and hypomethylating agent (HMA) failure higher risk myelodysplastic syndrome (MDS) have limited treatment options and time to therapy is of critical importance. PRGN-3006 UltraCAR-T cells express a CD33 chimeric antigen receptor (CAR), membrane bound IL-15 (mbIL15) and a kill switch with robust activity in preclinical models. UltraCAR-T cells are manufactured from autologous T cells via a decentralized overnight manufacturing process at medical centers without ex vivo activation or expansion, allowing infusion the day after gene transfer.
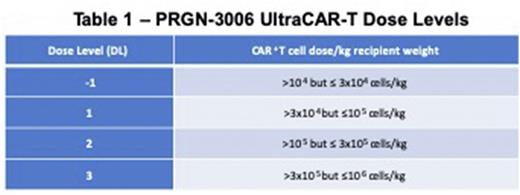
Methods: The Phase 1/1b study of PRGN-3006 UltraCAR-T evaluates the safety, in vivo expansion and effectiveness of PRGN-3006 in adult patients (pts) with r/r AML or HMA failure higher risk MDS or chronic myelomonocytic leukemia (CMML) with ≥ 5% blasts (NCT03927261). Pts received PRGN-3006 infusion without (Cohort 1, C1) or with lymphodepletion (fludarabine 30mg/m2 and cyclophosphamide 500mg/m2 days -5 to -3; Cohort 2, C2). Dose escalation of Cohorts 1 and 2 occurred in parallel with initial clearance of dose in Cohort 1 (Table 1).
Results: As of July 15, 2022 data cut-off, 24 pts have been treated in C1 (n=10) and C2 (n=14). The study enrolled 20 r/r AML, 1 CMML, and 3 MDS pts with a median age of 60.5 years (33-77). Pts were heavily pre-treated with a median of 3 prior regimens (range, 1-9) and 62.5% of pts (n=15) had prior allogeneic hematopoietic cell transplantation (alloHCT). Pts treated in C1 and C2 received 1.8 to 50 x 106 and 4.4 to 83 x 106 UltraCAR-T cells via intravenous (IV) infusion respectively.
PRGN-3006 infusion at up to 1x106 cells/kg was well tolerated with similar safety profile for pts treated in C1 and C2. There have been no deaths, dose-limiting toxicities (DLTs), or unexpected on-target/off-target toxicities related to PRGN-3006, and no use of the kill switch as of data cut-off. There have been no cases of bone marrow aplasia. Grade 1 cytokine release syndrome (CRS) occurred in 3/10 pts in C1 ((DL1 (n=1), and DL3 (n=2)), and 7/14 pts in C2 ((DL1 (n=1), DL2 (n=2) and DL3 (n=4)). Grade 2 CRS occurred in 3/10 pts in C1 ((DL1 (n=1), and DL3 (n=2)) and 3/14 pts in C2 (DL3 (n=3)). Only 1 pt (DL1, C1) had transient grade 3 CRS that resolved in < 24 hours with tocilizumab and dexamethasone. Plasma levels of IL-15 did not increase with treatment confirming mbIL15 is not shed.
A dose dependent expansion of PRGN-3006 was observed in peripheral blood and bone marrow in both C1 and C2 patients with durable in vivo persistence of UltraCAR-T in the blood for up to 7 months post infusion. Peak expansion in the blood was significantly higher (> 1 log10) in pts treated in C2 with lymphodepletion compared to C1 without lymphodepletion at the same dose level, with the highest peak expansion observed in subjects treated in Cohort 2 at DL3. UltraCAR-T cells expanded in the bone marrow of pts treated in C1 and C2 with the highest peak bone marrow expansion in pts treated in C2 at DL3.
Bone marrow blasts were reduced following treatment in 7/14 (50%) of lymphodepleted pts (C2), with 4 pts experiencing a decrease to ≤5%. AML pts in Cohort 2 (n=10) demonstrated a 30% objective response rate: 1 CRi was bridged to alloHCT and remains in a measurable residual disease negative CR 18 months post-transplant; 1 CRh with complete cytogenetic remission and NGS clearance; and 1 PR in a pt with isolated extramedullary leukemia. No objective responses were observed in CMML (n=1) or MDS (n=3) pts treated to date on the study. In non-lymphodepleted pts (C1), 1/10 pts had durable stable disease lasting > 7 months with persistence of UltraCAR-T cells.
Conclusion: Administration of PRGN-3006 UltraCAR-T cells targeting CD33, without or with lymphodepletion, have been well tolerated with low grade CRS. PRGN-3006 UltraCAR-T expressing mbIL15 demonstrated a dose-dependent robust expansion and durable persistence in blood and bone marrow with or without lymphodepletion. Encouraging objective responses have been observed in AML patients treated with lymphodepletion. Complete Phase 1 dose escalation data along with updated safety, efficacy, PK/PD and cytokine data will be presented at the meeting. The Phase 1b expansion dose will be DL3 with lymphodepletion.
Disclosures
Sallman:Takeda: Consultancy; Agios: Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nemucore: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Incyte: Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Lixte: Patents & Royalties: LB-100; Intellia: Membership on an entity's Board of Directors or advisory committees. Elmariah:Bristol Myers Squibb: Research Funding. Sweet:Syntrix Pharmaceuticals: Research Funding; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Chakaith:Precigen: Current Employment, Current equity holder in publicly-traded company. Semnani:Precigen: Current Employment, Current equity holder in publicly-traded company. Shehzad:Precigen: Current Employment, Current equity holder in publicly-traded company. Anderson:Precigen: Current Employment, Current equity holder in publicly-traded company. Sabzevari:Kinnate Biopharma: Membership on an entity's Board of Directors or advisory committees; Precigen: Current Employment, Current equity holder in publicly-traded company. Lankford:Precigen: Current Employment, Current equity holder in publicly-traded company. Chan:Syntrix Pharmaceuticals: Research Funding. Padron:Blueprint: Honoraria; Incyte: Research Funding; Taiho: Honoraria; Stemline: Honoraria; Kura: Research Funding; Syntrix Pharmaceuticals: Research Funding; BMS: Research Funding. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Komrokji:CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; Acceleron Pharma: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Lancet:Dedham Group: Consultancy; Agios/Servio: Consultancy; Astellas: Consultancy; Jasper Therapeutics: Consultancy; Novartis: Consultancy; Dava Oncology: Consultancy; Jazz: Consultancy; Boxer Capital: Consultancy; Syntrix Pharmaceuticals: Research Funding; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy; Servier: Consultancy. Davila:Bellicum: Consultancy; Kite: Research Funding; Novartis: Research Funding; Synthekine: Consultancy; Adicet: Consultancy; CRISPR: Other: licensing fees, Research Funding; Atara: Other: licensing fees, Patents & Royalties: WO2019165156, Research Funding. Bejanyan:Medexus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; CareDX Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal